How a Water Softener Works

Hard water is a generic term used to refer to a water supply that has absorbed high quantities of minerals from the earth—primarily calcium and magnesium. Water is described as "soft" if it lacks these minerals in substantial quantities. Water can be naturally soft (water supplies derived from lakes and streams are often naturally soft). Or it can be soft due to artificial treatment, either at the municipal water utility company or by means of an in-house water treatment appliance known as a water softener.
What Is a Water Softener?
A water softener is a type of filtering appliance that removes calcium and magnesium from the water.
Underground water supplies become hard because of the nature of the rock through which rainwater percolates as it filters down from the surface. Regions that are high in limestone, chalk, and gypsum usually have hard water in their groundwater reservoirs, while regions where the predominant rock is granite or another low-calcium stone have naturally soft water. Water can also become hard in regions where certain agricultural amendments such as lime are used in large quantities.
Sulfur content is another issue that can be treated as well. The obvious signs of too much sulfur in the water are a bad odor and water that is a brown or reddish color. This too can lead to corrosion or bacteria in your drinking water.
Problems Caused by Excessively Hard Water
There is no health concern with drinking hard water high in dissolved minerals such as calcium and magnesium. If fact, small amounts of these minerals are necessary for health. But at high levels the minerals can build up in pipes, plumbing fixtures, and appliances, gradually interfering with their function. For example, mineral scale buildup in faucets or water heaters may shorten their lifespan. Some people report feeling itchy after bathing or showering in water that is very high in mineral content.
Hard water also reduces the ability of soap to lather, and the minerals can combine with the soap to form a sticky scum that clings. If you have problems with water spots on dishes, for example, it may indicate you have a hard water problem. This sticky scum can also be hard to rinse out of clothes or your hair.
Types of Water Softeners
Water softeners come in several types:
- Ion exchange: This is by far the most common type of water softener in home application. It works by removing calcium and magnesium ions and replacing them with sodium ions, which have none of the damaging effects of calcium and magnesium. This is the familiar device that includes a large tank of salt pellets. If your home has a water softener, it is very likely this type.
- Salt-free: This device uses a mechanical filter to remove calcium, but it doesn't work very well on very hard water. It does not remove magnesium.
- Reverse osmosis: This device filters water through a semipermeable membrane that removes as much as 98% of water impurities.1 It is an expensive appliance, and it uses a considerable amount of water. But this type of device is very good at removing other chemical impurities, as well as calcium and magnesium. However, the use of reverse osmosis for drinking water can have negative effects. The human body needs minerals, derived from drinking water among other sources. Long term use of reverse osmosis can have unintended consequences and actually deplete the human body of important minerals
Do I Need a Water Softener?
The water softener industry spends millions of dollars to convince consumers that water softeners are absolutely necessary for every home. The reality, though, is that water softeners may not be necessary in many areas. Most state environmental agencies recommend that unless your water hardness level exceeds 7 parts per gallon (ppg), or 120 mg per liter, you really don't need a water softener.2 The official USGA map of water hardness indicates that more than half of the U.S. falls into this category with the the highest concentrations in the Great Plains, Rocky Mountain, and Midwest states. However, even if you live in a region with hard groundwater, there is a good chance that your local water utility already treats the water to reduce hardness. And if your community takes its water supply from a river or lake, there is a good chance it is already relatively soft, even when underground water supplies are hard. Most of the time, hard water of high mineral content comes from well water, which is untreated municipal water.
Water softener manufacturers, predictably, claim that it is a serious problem to have any trace of mineral hardness in water, and they argue that even"slightly hard" and "moderately hard" water should also be softened. They will argue that any water hardness over 1 ppg requires softening. Ultimately, this is a matter of personal preference, but be aware that the official stance is that only distinctly hard water (7 parts per gallon or more) requires softening.
Pros and Cons of Water Softening
The choice of whether or not to soften your water is based on how you value the various advantages and disadvantages:
Advantages to Using a Water Softener:
- Eliminates buildup of scale on dishes, pipes, plumbing fixtures, and appliances; may lengthen their lifespan. Softened water allows soaps and detergents to work more effectively.Softened water is more comfortable on the skin for some people.
Disadvantages of Using a Water Softener:
- Small, but notable health risk for people on low-sodium diets. The ion exchange process adds roughly 7.5 mg per quart of water. Removes virtually all calcium and magnesium from water, which for some people may require dietary supplements to replace these necessary elements. Ongoing maintenance costs and chores. Salt must be periodically added to the brine tank, and the device needs to be periodically serviced. Some people find the feel of soft water to be slippery and slimy. The process discharges salty water into the sewer system, posing potential environmental problems. Softened water contains a small amount of sodium that can cause problems with septic systems.
Problems a Water Softener Won't Fix
Water softeners are good at removing calcium and magnesium, but they don't do anything to remove other minerals and gases that can cause problems in drinking water. For example, ferrous iron is a common mineral and it creates nasty rust stains in sinks, tubs, and toilets. And manganese causes black staining and is often found with iron. Water that has a faint “rotten egg” smell has hydrogen sulfide gas dissolved in it, and a water softener does nothing to remove this smell. You must have a water treatment system designed for eliminating these additional minerals—a water softener won't remove them.
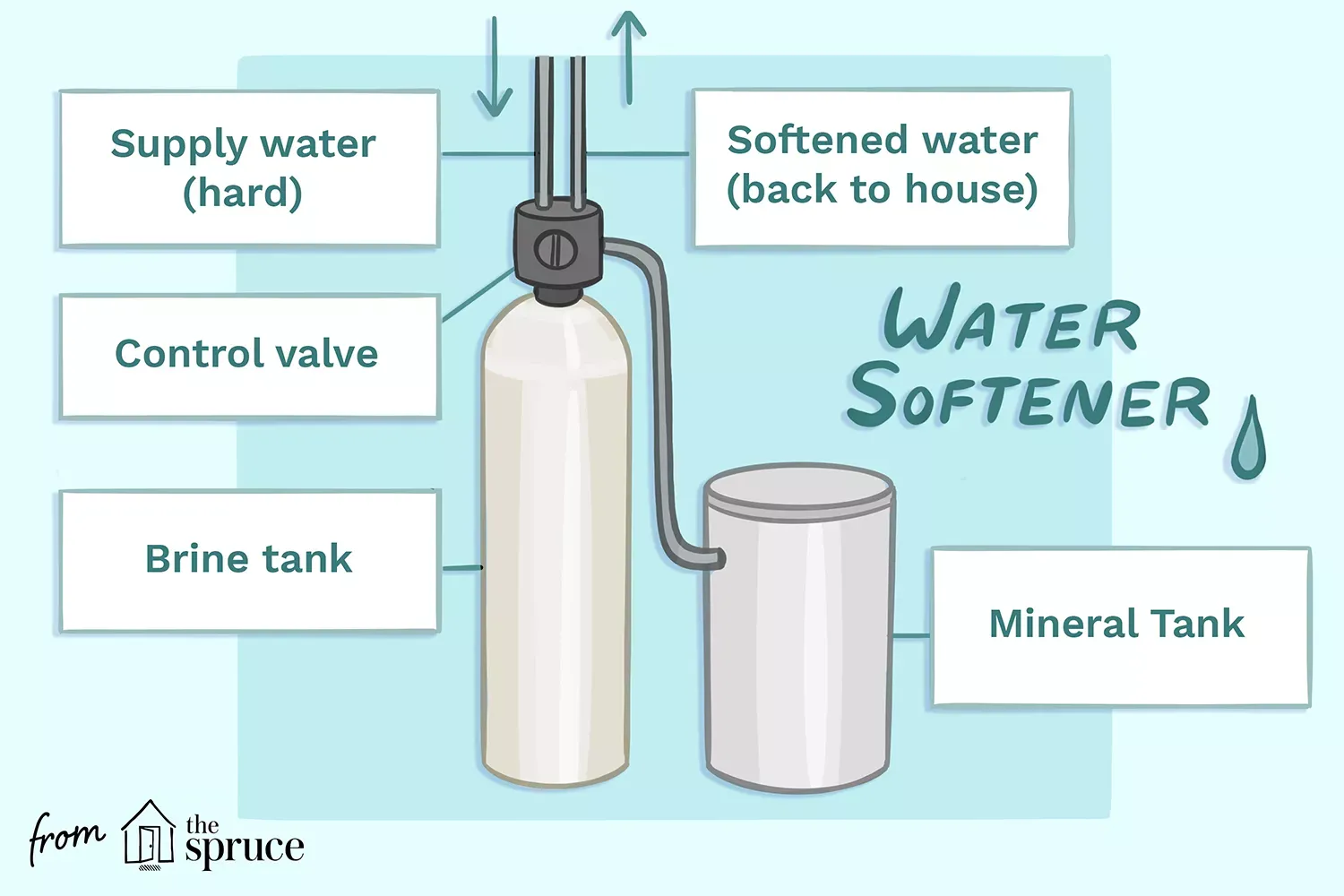
The Parts of an Ion-Exchange Water Softener
By far the most common type of residential water softener is the ion-exchange system. Understanding the function of each part will help you maintain it correctly.
Ion-exchange water softeners have three main components: a mineral tank, brine tank, and a control valve. Smaller capacity models combine the mineral tank and brine tanks into one cabinet, but the two tanks are still separated within the cabinet.
Larger flow capacity systems have a separate stand-alone mineral and brine tanks:
- Mineral tank: The mineral tank is where the action is. It is where the water filtration takes place and the hard water is softened by removing calcium and magnesium.
- Brine tank: The brine tank is where a highly concentrated solution of salt or potassium is stored. This brine solution comes into play to flush the mineral tank and recharge it. The brine tank must be periodically replenished with salt or potassium pellets.
- Control valve: The control valve is the device that controls the flow of water into and out of the mineral and brine tanks during regeneration.
In most instances, a water softener is located near the point where the water supply enters the house, and is installed so it treats the water used for drinking, cooking, and washing, but not the water used for outdoor irrigation.
The Mineral Tank
The mineral tank is the tall narrow tank where the actual water softening occurs. It is filled with several cubic feet of porous plastic polystyrene resin beads. As water flows through this tank, the negatively charged beads attract and hold the positively charged calcium and magnesium particles in the water. With these hard minerals trapped by the resin beads, the water that flows onward is now soft.
Eventually, though, the beads become saturated with minerals, and will need to be cleaned (regenerated). The next components of the system are integral to that process.
The Brine Tank
The brine tank is just what its name suggests: a plastic tank that contains brine—water saturated with salt or potassium. This salty water will be used when it comes time to backwash the mineral tank, remove the mineral particles, and restore the negative charge to the beads so that they can continue to trap more minerals.
Normally, the brine tank is kept filled with salt (sodium). But problems may result if you are on a restricted low-sodium diet, since a small amount of sodium is transmitted into the drinking water during the regeneration process. And sodium can also affect the bacteria in septic systems that are necessary for the breakdown of waste. For this reason, sodium brine has been banned or limited in several states and municipalities such as California.
As an alternative, potassium may be used for the brine. Potassium is considered superior and environmentally friendly, albeit a bit more expensive, than salt, and it does not affect your health, watersheds, or the function of septic systems.
The Control Valve and the Regeneration Process
The control valve serves as the traffic cop in your water softener system. It determines when it is time to clean those plastic beads, which are now coated with calcium and magnesium. Older style units use a timer, while newer models use a computer-controlled meter that determines when it is time for regeneration, based on actual water usage.
The Regeneration Process
To clean the beads in the mineral tank, the water softener uses a process often called regeneration, which consists of three cycles: backwash, recharge, and rinse. This process occurs every few days, and normally is initiated in the middle of the night.
Backwash: Regeneration starts with a backwash cycle in which the valve reverses the water flow in the tank and flushes the tank of debris. This debris is then eliminated through the drain connected to the municipal sewer system or septic system.
Recharge (regeneration): In the recharge cycle, the salty solution is pumped from the brine tank into the mineral tank. The highly concentrated salt solution forces the magnesium and calcium off the beads, and the mineral-rich salty water is then flushed out of the tank and down the drain.
Rinse: The mineral tank is then filled and rinsed with water, the regeneration process is halted and the water softening process repeats itself.
In the freshly regenerated mineral tank, the beads are now coated with sodium or potassium provided by the brine tank. As additional hard water enters the mineral tank, the positively charged calcium and magnesium in the water are attracted to the plastic beads, replacing the sodium on the beads. This small amount of salt displaced from the beads becomes suspended in the water and moves on into the home water supply. This small amount of salt in the water is generally not a problem, except for people who have strict limitations on sodium.
When the beads again become saturated with hard-water calcium and magnesium, the control valve starts a new regeneration cycle and flushes the hard-water minerals down the drain once more. This ongoing cycle continues so long as the brine tank is kept stocked with salt or potassium pellets.
Source: https://www.thespruce.com/water-softeners-how-they-work-1824916

















Share On: